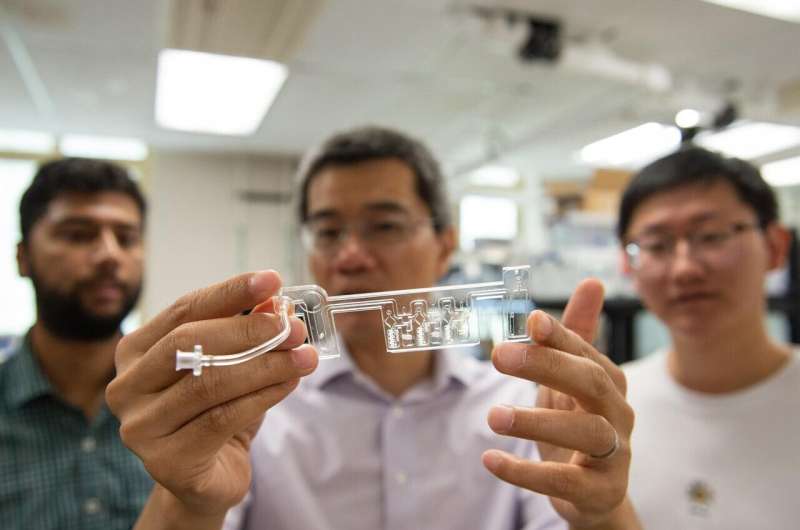
Penn State researchers developed a home saliva-based testing platform that can provide results in as little as 45 minutes. In preliminary tests, the platform detects the COVID-causing virus with the same sensitivity as PCR tests. Credit: Kelby Hochreither, Penn State
PCR testing, also known as molecular testing or nucleic acid testing, is considered the gold standard in detecting the presence of SARS-CoV-2, the virus that gives rise to COVID-19. However, they can take a few days to process resulting in unnecessary quarantine for negative individuals or delays for those who need proof of negative tests for travel or other commitments. Rapid antigen detection tests, on the other hand, are convenient but less reliable than PCR tests.
To bridge the gap between accuracy and convenience, researchers at Penn State have developed a home saliva-based testing platform that can provide results within 45 minutes. In preliminary tests, the platform has detected the COVID-causing virus with the same sensitivity as PCR tests. Their results were published this week in ACS sensors.
“PCR test results take about an hour to develop in a lab, but you have to factor in the time it takes to send the sample to a lab and for the lab to process it,” said lead researcher Weihua Guan , associate professor of electrical engineering and of biomedical engineering in Penn State’s College of Engineering. “We wanted to create a viable alternative to the PCR for people to use at home, without having to go through the invasive nasal test.”
Guan and his team developed a palm-sized test kit in which a person spits into a cartridge and inserts it into the processing platform. Within 45 minutes, the test results are sent to a custom Android app developed by the researchers.
The platform uses reverse transcription loop-mediated isothermal amplification, or RT-LAMP, to detect the virus. The test device first heats the saliva to 203 degrees Fahrenheit, the temperature at which the shells of viral particles break apart and release their genetic material. The genetic material is then mixed with prepackaged reagents in a microfluidic cartridge. Finally, the sample is cooled to 149 degrees Fahrenheit, triggering a new chemical reaction that multiplies a few viral molecules into billions of copies, making the virus easier to identify. If the virus is present in the saliva sample, the user will get a positive result on their connected smartphone app.
To test the setup, Guan and his team infused human saliva samples purchased commercially with inactivated SARS-CoV-2 virus particles and ran the samples through the prototype. They also tested a number of clinical specimens. The platform accurately determined whether each sample was positive or negative for the virus.
“We tested hundreds of fake samples and checked the amount of COVID particles in each one,” Guan said. “Our platform was found to be highly sensitive to the presence of the virus in both the fake and clinical samples, using the standards established by the PCR test as our benchmark.”
Electrical engineering doctoral students Aneesh Kshirsagar (left) and Tianyi Liu (right) were part of the project team that developed the RT-LAMP test, led by lead researcher Weihua Guan, associate professor of electrical engineering and biomedical engineering (center). Two Penn State electrical engineering alumni, Tianyi Liu and Jiarui Cui (not pictured), were also critical members of the team. Credit: Kelby Hochreither, Penn State
The researchers said they plan to continue testing their platform with more clinical COVID samples through a collaboration with Yusheng Zhu, medical director of the Clinical Chemistry and Automated Testing Laboratory at Penn State Milton S. Hershey Medical Center.
In addition to more clinical trials, the researchers are also working to improve the short shelf life of the test, as the enzymes in the prototype degrade within three days of production at room temperature. The team is experimenting with reagent freeze-drying, a method of freeze-drying biological material that can extend the shelf life of enzymes. According to Guan, preliminary results indicate that the method allows the RT-LAMP test to last at least six months at room temperature in stores or in the home medicine cabinet.
The researchers filed a preliminary patent application for their device and said they plan to commercialize it, pending scaled-up clinical testing and review by the U.S. Food and Drug Administration.
Suresh V. Kuchipudi, interim director of Penn State’s Animal Diagnostic Laboratory (ADL) and Dorothy Foehr Huck and J. Lloyd Huck Chair in Emerging Infectious Diseases, and Michele Yon, research technologist at ADL, obtained the clinical samples featured in this work. tested. Other contributors include Zifan Tang, Aneesh Kshirsagar, and Tianyi Liu, all Penn State doctoral students in electrical engineering, and Jiarui Cui, an electrical engineering student at Penn State.
Zifan Tang et al, SLIDE: saliva-based SARS-CoV-2 self-test with RT-LAMP in a mobile device, ACS sensors (2022). DOI: 10.1021/acssensors.2c01023
Quote: New home saliva-based COVID test as effective as PCR in preliminary analysis (2022, Aug 5) retrieved Aug 5, 2022 from https://phys.org/news/2022-08-at-home-saliva-based -covid-effective-pcr.html
This document is copyrighted. Other than fair dealing for personal study or research, nothing may be reproduced without written permission. The content is provided for informational purposes only.